How to Write Ionic Equations
Free-body diagrams showing these forces their direction and their relative magnitude are often used to depict such information. Ionic vs molecular equation form.

How To Write The Net Ionic Equation For Hcl Zns H2s Zncl2 Chemical Equation How To Find Out Math
Ionic chemical equations are slightly different from that of a classic case of chemical equations.

. Estimate the solubility of Ag 2 CrO 4 in pure water if the solubility product constant for silver chromate is 11 x 10-12. Alan Hodgkin and Andrew Huxley described the model in 1952 to explain the ionic mechanisms underlying the initiation and propagation of action potentials. CaS H 2 O CaO H 2 S.
When a solute is mixed with a solvent there are three possible outcomes. You need to enable JavaScript to run this app. Write balanced formula unit total ionic and net ionic equations for the reaction Question 14A solution of AgNO 3 is mixed with a solution of K 2 S.
Ionic chemicals involve electrolytes which are substances that dissociate into ions when dissolved in polar solvents. If a question is asking about the high melting point of magnesium oxide a student will not get much credit for just saying it has strong bonds or strong ionic bonds. What makes a wave a wave.
Write a balanced half equation for the formation of calcium from a calcium ion. Fourth substitute the equilibrium concentrations into the equilibrium expression and solve for K sp. Examples and equations may be included in your responses where appropriate.
Calculating the Solubility of an Ionic Compound in Pure Water from its K sp. A balanced ionic equation. 3 Group II sulfates become less soluble down the group.
The correct formulas of the reactants are always written on the left side of the equation. Write the equation and the. When an equation is written in the molecular form the program will have issues balancing atoms in parcial equations of oxidation and reduction Step 3.
The ions are forced to undergo either oxidation at the anode or reduction at the cathode. HNO 3aq NaOH aq NaNO 3aq H 2 O l 2. Several examples are discussed.
In this Lesson The Physics Classroom discusses the details of constructing free-body diagrams. Four convention are used to write chemical equations. Electrolysis involves passing an electric current through either a molten salt or an ionic solution.
Therefore decomposition reactions are. A chemical equation is the symbolic representation of a chemical reaction in the form of symbols and formulae wherein the reactant entities are given on the left-hand side and the product entities on the right-hand side with a plus sign between the entities in both the reactants and the products and an arrow that points towards the products and shows the direction of the reaction. The motion of objects is determined by the relative size and the direction of the forces that act upon it.
MnO 4- Mn 2 I- I 2. For example the ionic equation. If there is more solute than is able to be dissolved the excess separates from the.
Figure 11 Chemical substances and processes are essential for our existence providing sustenance keeping us clean and healthy fabricating electronic devices enabling transportation and much more. BaSO4 is the least soluble. The other is the evaluation of the energy in different ways.
Modification of work by vxlaFlickr. 2H aq Mgs Mg 2 aq H 2 g This ionic equation can be split into two half equations. Write a skeleton ionic equation that only covers the atoms that change oxidation number.
Here are the steps involved in balancing chemical equations plus a worked example. Write equations for these reactions. Write the molecular total ionic and net ionic equations illustrating the reaction.
In aqueous solutions its common to balance chemical equations for both mass and chargeBalancing for mass produces the same numbers and kinds of atoms on both sides of the equation. One is the application of the concept of energy to electrostatic problems. Convention used in Writing Chemical Equations.
If the solution has less solute than the maximum amount it is able to dissolve the solubility it is a dilute solutionIf the amount of solute is exactly the same as the solubility it is saturated. How can waves be described in a manner that allows us to understand their basic nature and qualities. Being able to balance chemical equations is a key chemistry skill.
It is a continuous-time dynamical system. This is avoided by writing the equation in the ionic form. CaS H 2 O CaO H 2 S.
Modification of work by the Italian voiceFlickr. You need to enable JavaScript to run this app. MgO has a giant lattice structure with strong electrostatic forces of attraction between oppositely charged ions.
According to the equations three moles of electrons produce one mole of iron and 2 moles of. For the reaction of magnesium with hydrochloric acid is. In this Lesson the nature of a wave as a disturbance that travels through a medium from one location to another is.
Write the ionic equation for the word equation. K sp 0015900318 2 161 x 10-5. Balancing for charge means the net charge is zero on both sides of the equation.
Shows the reacting ions. In a chemical reaction. Write the balanced half-reactions involved.
We shall concern ourselves with two aspects of this energy. Sometimes it is easier to compute the work done for some. The ionic equations can be represented by two half equations.
Testing for Presence of a Sulfate ion BaCl2 solution acidified with hydrochloric acid is used as a reagent to test for sulphate ions. Example of Balanced Ionic Equation. Write the balanced net ionic equation for the reaction that occurs when H.
Scroll down the page for more examples and solutions on writing ionic equations. Balanced ionic equations - Higher. These require a lot of energy to break.
The following diagram shows how to write the ionic equation for the reaction of aqueous sodium carbonate with aqueous barium nitrate. Question 15Write decomposition reactions for the following compounds aCaHCO 3 2 bAg 2 O cN 2 O 3 Question 16. What characteristics properties or behaviors are shared by the phenomena that we typically characterize as being a wave.
In a decomposition reaction a single substance breaks down into two or more substances while in a combination reaction two or more substances react to produce one substance. The correct formulas of the products are always written on the right side of the equation. Balance all of the atoms besides the oxygen O.
If acidified barium chloride is added to a solution that contains sulfate ions a white precipitate of barium sulfate forms. This worksheet will help you practise writing ionic equations for neutralisation and precipitation reactions Where state symbols are not given youll need to use the solubility rules to determine whether a substance will ionise Write ionic equations for the following. You must show your work to receive credit for your answer.
These electrolytes are split up and written as individual ions when written in ionic chemical equations. For calculations clearly show the method used and the steps involved in arriving at your answers. It is a set of nonlinear differential equations that approximates the electrical characteristics of excitable cells such as neurons and muscle cells.
Modification of work. A good answer might be.

Net Ionic Equation Worksheet And Answers Youtube Equations Chemistry Study Tips
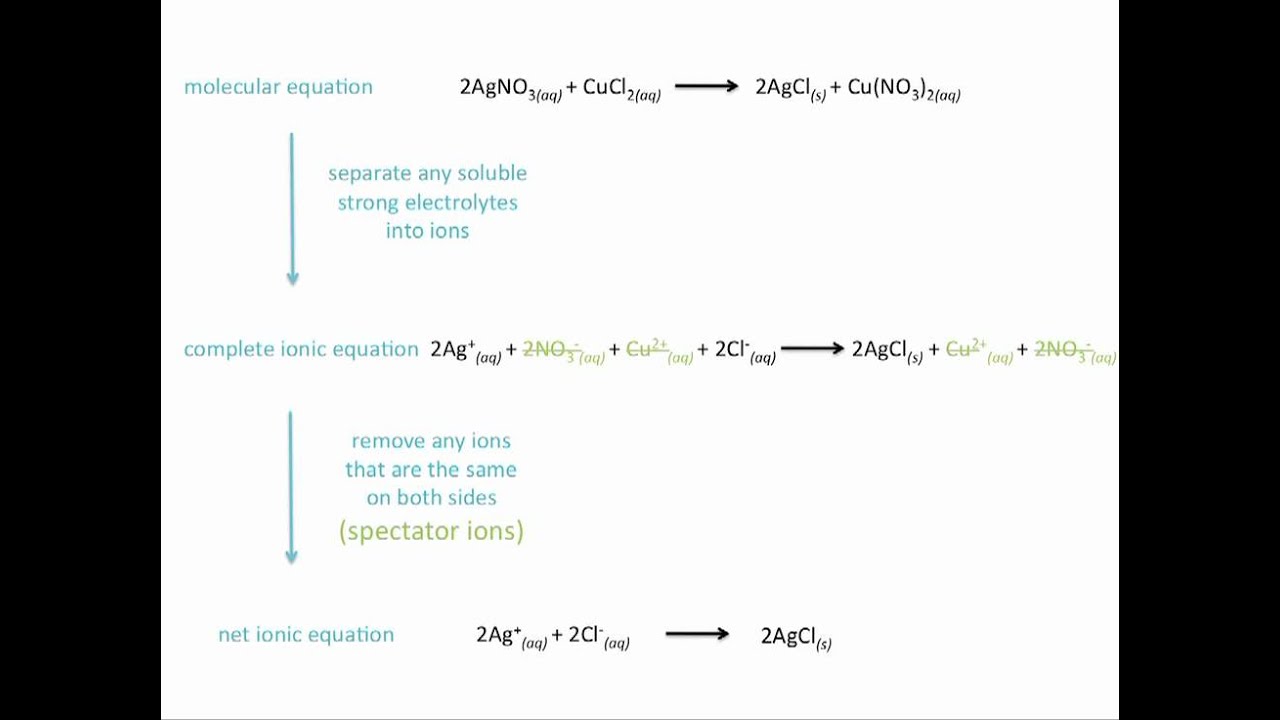
Ionic Equations Net Ionic Equations And Spectator Ions Chemistry Tutorial Chemistry Equations Ionic

Net Ionic Equations Ap Chem Chemistry Class Equations

How To Write Ionic Equations Tutor Pace Science Chemistry Chemistry Class Chemistry Notes
0 Response to "How to Write Ionic Equations"
Post a Comment